Table of Contents
Object:
To synthesize and submit 1-Phenylazo-2-naphthol
References:
Mann, F. G.; Saunders, B. C., “ Practical Organic Chemistry”, 4th edition (Reprint 1978) Longman Group Ltd., London, Page no-210-211.
Chemical requirement :
Aniline (freshly distilled): 4.0g
Hydrochloricacidconc.(12N) : 12.8ml
β-Naphthol: 6.24 g
Sodium hydroxide solution [10%(w/v)] : 40ml
Sodium nitrite (pure) : 3.2g
Theory:
Phenyl diazonium chloride is obtained first by the diazotization of aniline with nitrous acid, which on coupling with β-naphthol in the presence of NaOH solution yields the desired coupled product phenyl-azo-β-naphthol. A mole of HCl is eliminated which instantly reacts with NaOH from the medium to produce NaCl and H2O. Importantly, both diazotization and coupling reactions are required to be carried out at between0-5°C.
Procedure:
- In a 250 ml beaker dissolve 4.0g (3.92ml;0.054mol) of aniline in 12.8ml conc. HCl and dilute it with 12.8 ml distilled water. Cool the contents of the beaker in an ice bath with frequent stirring till it attains a temperature between 0-5°C.[One may observe that the freshly distilled oily aniline has completely dissolved in the aqueous medium as aniline hydrochloride.]
- Meanwhile, dissolve separately 3.2 g sodium nitrite in 15ml water and chill the solutional so in the same ice-bath (0–5°C).
- Diazotise the aniline solution
(1) by the addition of sodium nitrite solution
(2) in small lots (2ml) at a time in intervals with vigorous stirring with a glass rod taking care that the temperature of their action mixture must not exceed beyond 5° C at any cost. (If required 10-15 g of crushed ice may be added into the reaction mixture to ensure proper chilling while diazotization is on). - After the complete addition of sodium nitrite solution, it is required to test their action mixture for the presence of free nitrite by taking out a drop of it and immediately placing it on KI-starch paper that will distinctly turn blue in the presence of free nitrous acid. (It may be noted that by using good quality sodium nitrite and adding 10% excess than the theoretical value one may ascertain completion of diazotization reaction).
- Dissolve 6.24g (0.054mol) β-naphthol separately in a 250ml beaker in 40ml of sodium hydroxide solution, and cool the naphthol-solution in an ice-bath(0-5°C).
- Cautiously and slowly add the cold diazonium salt solution to the β-naphthol solution with vigorous constant stirring. Special care must be taken for not allowing the temperature of the reaction mixture to rise beyond 5°C. If need be, crushed ice should be added in between while the coupling-reaction proceeds.
- Red color develops and crystals of crude phenyl-azo-β-naphthol separate out. Allow the reaction mixture to stand for 30-40 minutes with stirring in between so as to complete the reaction.
- FiltertheredproductinaBüchnerfunnelusingsuction, and wash the same with ice-cold water. Drain the water by pressing with an inverted glass-stopper.
- mp= 129-130°C
Chemical reaction involved:

Calculation:
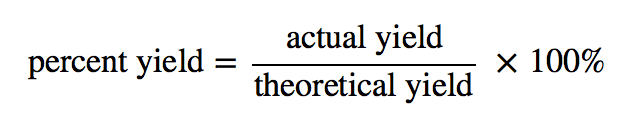
Result:
The deep red crystals of 1-Phenylazo-2-naphthol are obtained.
% yield was found to be:……………
The melting point was found to be:…………..