Table of Contents
Object:
To synthesize and submit salicylic acid by hydrolysis of aspirin.
References:
Mann, F. G.; Saunders, B. C., “ Practical Organic Chemistry”, 4th edition (Reprint 1978) Longman Group Ltd., London, Page no-111.
Chemical requirement:
Aspirin,sodium hydroxide solution,dil.sulphuricacid
Theory:
Aspirin can under-go hydrolysis making it not as effective if it’s exposed to water for extended periods of time. The technical name of the active ingredient in aspirin is acetylsalicylic acid. When it reacts with water, we end up with two products, salicylic acid, and acetic acid. This reaction can occur with acidic or basic conditions.
Let’s first take note of the ester on aspirin
CH3COOC6H4COOH or C9H8O4

- Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion.
- The negatively charged hydroxide attacks the carbonyl carbon of the acetate group resulting in hydrolysis of aspirin into salicylate and acetate(general base catalysis).

Procedure:
• Gently boil a mixture of 1g of aspirin and 15ml of 10% sodium hydroxide solution in a 50ml conical flask under reflux for 20minutes.
• Then cool the solution thoroughly and add dilute sulphuric acid until the precipitation of the salicylic acid is complete. Filter off the salicylic acid, recrystallize it from hot water and confirm its identity by reaction (i)above.M.p.156°C.
• The original filtrate still contains the acetic acid which is the other product of the hydrolysis. Therefore place this filtrate(which must be distinctly acid to litmus-paper)in a distilling-flask and distill off about 5ml through a water-condenser. Then apply to the filtrate the tests for acetic acid.
Chemical reaction involved :

Calculation:
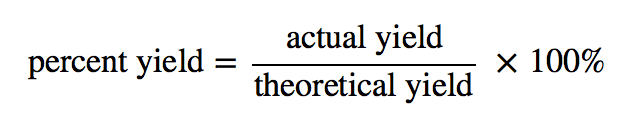
Result:
The crystals of salicylic acid were obtained.
% yield was found to be:…………….
The melting point was found to be:…………..








Hey there would you mind stating which blog platform you’re working with? I’m going to start my own blog in the near future but I’m having a hard time making a decision between BlogEngine/Wordpress/B2evolution and Drupal. The reason I ask is because your design seems different then most blogs and I’m looking for something completely unique. P.S My apologies for getting off-topic but I had to ask!
mail us at admin@recnotes.com for more information.