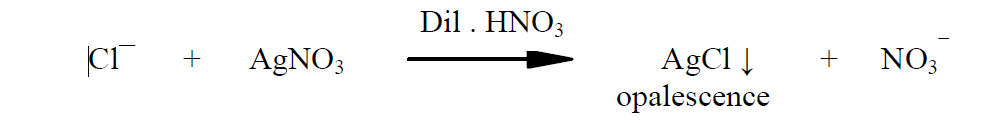Table of Contents
Aim:
To perform limit test for chlorides for the given sample.
Requirements:
Nessler cylinders, glass rod, measuring cylinders, 1ml bulb pipette, 10 ml bulb pipette, dilute nitric acid, 0.1 M silver nitrate, chloride standard solution (25 ppm Cl), test sample.
Principle:
In this experiment, the test opalescence obtained by the reaction of chloride impurities with silver nitrate is compared with standard opalescence obtained by the reaction of known quantity of chloride with
silver nitrate. Dilute nitric acid is used to dissolve other impurities if present.
The precipitate silver chloride formed is insoluble in dilute nitric acid and
gives opalescence.
Procedure:
Test opalescence:
Dissolve the given sample in 20 ml of water and transfer to a Nessler cylinder. Add 10 ml of dilute nitric acid, dilute to 50 ml with water. Add 1 ml of 0.1 M silver nitrate. Stir immediately with a glass rod and allow to stand for 5 minutes, protected from light. View transversely against a black background.
Standard opalescence:
Transfer 10.0 ml of chloride standard solution (25 ppm Cl) into a Nessler cylinder and add 5 ml of water. Add 10 ml of dilute nitric acid, dilute to 50 ml with water. Add 1 ml of 0.1 M silver nitrate. Stir immediately with a glass rod and allow to stand for 5 minutes, protected from light. View transversely against a black background.
|
Test Solution |
Standard Solution |
cylinder. |
|
|
|
|
|
|
|
Observation:
Test opalescence is not more intense than standard opalescence.
or
Test opalescence is more intense than standard opalescence.
Report/Result:
The given sample passes limit test for chlorides.
or
The given sample fails limit test for chlorides.
Preparation of Reagents:
1. 0.1 M silver nitrate: Dissolve 17.0 g of silver Nitrate in sufficient water to 1000 ml. (Store in light-resistant containers)
2. Chloride standard solution (25 ppm Cl): Dilute 5 volumes of a 0.0824% w/v of sodium chloride to 100 volumes with water.
3. Dilute nitric acid: Contains approximately 10% w/w of HNO3. Dilute
106 ml of nitric acid to 1000 ml with water.
Also Read ;