Table of Contents
Objectives:
- To understand the importance of measuring blood glucose level.
- To understand the principles of enzymatic estimation of glucose.
Theory:
Glucose is a simple sugar which is a permanent and immediate primary source of energy to all of the cells in our body. The glucose in blood is obtained from the food that you eat. This glucose gets absorbed by intestines and distributed to all of the cells in body through bloodstream and breaks it down for energy.
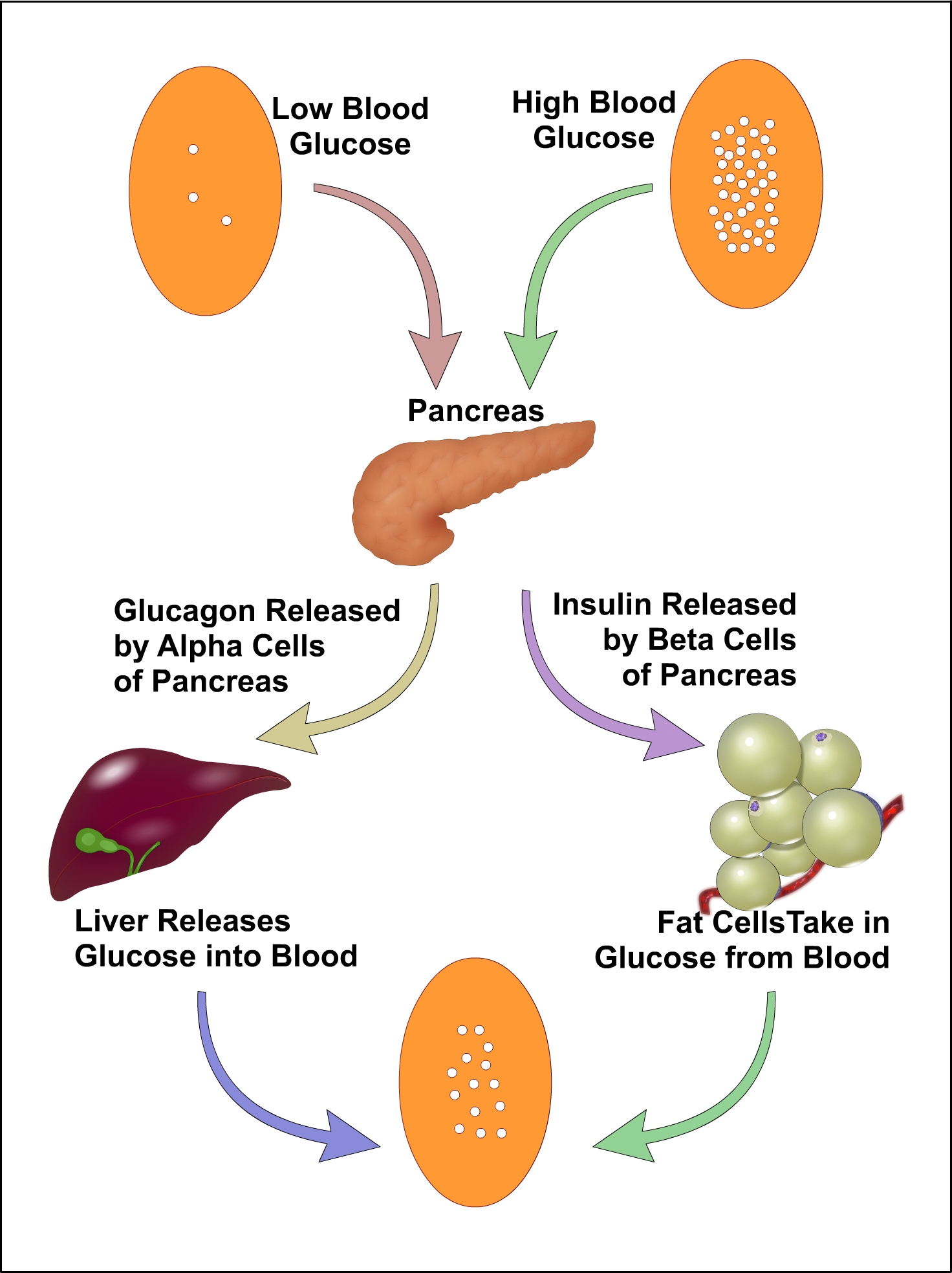
Body tries to maintain a constant supply of glucose for your cells by maintaining a constant blood glucose concentration. The concentration of glucose in blood, expressed in mg/dl, is defined by the term glycemia. The value of blood sugar in humans generally ranges from 70 – 100 mg/dl. Blood sugar levels are regulated by the hormones insulin and glucagon which act antagonistically. These two hormones are secreted by the islet cells of the pancreas, and thus are referred to as pancreatic endocrine hormones. When the blood glucose levels are high, insulin hormone secreted which causing liver to convert more glucose molecules into glycogen and when the blood glucose levels are low glucagon secreted and act on liver cells to promote the breakdown of glycogen to glucose and increases the blood glucose concentrations. Essentially blood glucose levels determine the time of secretion of these hormones.
The blood glucose level is easily changed under the influence of some external and internal factors such as body composition, age, physical activity and sex. Diabetes is a disease related by the abnormal metabolism of blood sugar and defective insulin production. So blood sugar levels are an important parameter for the study of diabetes. The level of glucose circulating in blood at a given time is called as blood glucose level. The blood glucose level varies at different time on various part of the day. Hypoglycemia is a possible side effect of diabetes medications in which blood glucose level drops below 70mg/dl. In people with diabetes, the body doesn’t produce enough insulin or respond to insulin properly. The result is that sugar builds up in the blood stream, damaging the body’s organs, blood vessels and nerves. This condition in which too much sugar in the blood stream is called hyperglycemia.
The blood glucose analysis is ordered to measure the amount of blood at the time of sample collection. It is used to detect both hyperglycemia and hypoglycemia and via helping the diagnosis of diabetes. An ideal blood glucose estimation method should determine only glucose. It is adaptable for both macro- and semi micro- techniques. Reagents are relatively inexpensive and the method should require a minimum of time, techniques and apparatus, be accurate and yield reproducible results. Glucose oxidase is an enzyme highly specific for glucose and is not react with blood saccharides. So it has been employed for the estimation of blood glucose.
Principle:
Glucose oxidase is an enzyme extracted from the growth medium of Aspergillus niger. Glucose oxidase catalyse the oxidation of Beta D- glucose present in the plasma to D glucono -1 ,5 – lactone with the formation of hydrogen peroxide; the lactone is then slowly hydrolysed to D-gluconic acid. The hydrogen peroxide produced is then broken down to oxygen and water by a peroxidase enzyme. Oxygen then react with an oxygen acceptor such as ortho toluidine which itself converted to a coloured compound, the amount of which can be measured colorimetrically.
Materials Required:
-
Collection of blood sample:
About 2ml of patient’s blood should be collected by venipuncture into a tube containing a mixture of ethylenediaminetetraacetic acid and sodium fluoride in the ratio of 1:2 (W/W). Five mg of the mixture is adequate for 2ml of blood. The tube should be thoroughly shaken for complete mixing.
-
Preparation of anticoagulant mixture:
100mg of EDTA and 20mg of sodium fluoride should be mixed and ground into a fine powder using a blender. This should preferably do in a fume hood. The mixture should be stored in a clean container.
Reagents:
- 2N Sodium hydroxide (NaOH) – 8g of NaOH is dissolve and finally make up the volume to 100ml with distilled water.
- Sodium Sulphate- Zinc sulphate reagent – Dilute 55ml of the zinc sulphate solution (10g/100ml ZnSO4.7H2O) to 1 litre with the sodium sulphate solution (93mmol/liter).
- Phosphate buffer 0.05M pH-7.2
- Glucose oxidase reagent: Prepare this reagent fresh by dissolving 25mg of glucose oxidase and 1% ortho – toluidine in the sodium phosphate buffer. Add a small quantity of peroxidase (2mg) and makeup to 250ml with the buffer. This solution is active for about 4 weeks if stored in a brown colored bottle at 4°C.
Procedure:
- Preparation of Test: Pipette 0.1ml of blood into 1.8ml of sodium sulphate-zinc sulphate reagent in a centrifuge tube. Add 0.1ml of 2N Sodium hydroxide, centrifuge at 3000rpm for 5 minutes and take 0.5ml of supernatant in duplicate.
- Preparation of Blank: Take 0.5ml of distilled water.
- Preparation of Standard: Prepare standard concentration of glucose (200mg/dl), use 0.5ml of a range of glucose solutions (50mg/dl, 100mg/dl, 150mg/dl and 200mg/dl) suitably diluted from standard.
I. 50mg/dl – 125µl glucose standard + 375µl distilled water
II. 100mg/dl – 250µl glucose standard + 250µl distilled water
III. 150mg/dl – 375µl glucose standard + 125µl distilled water
IV. 200mg/dl – 500µl glucose standard
- Add 5ml of the glucose oxidase reagent incubate for 1h at 37°C and read the extinction at 540nm against the reagent blank.
- If the absorbance reading of the sample is too high, dilute the supernatant which was obtained earlier, 2x with distilled water and repeat the subsequent step.
References:-
1. Trevor Palmer, Enzymes: Biochemistry, Biotechnology and Clinical Chemistry, 2nd edition.
2. David T Plummer, An Introduction to practical biochemistry, 3rd edition.
3. http://www.ncbi.nlm.nih.gov/books/NBK248/