Table of Contents
Our Objective
Our aim is to study the effect of different pH on the activity of salivary content, amylase on starch.
Theory
All living beings need the energy to survive. It is from the food we consume that we get our energy. We know that the energy we are getting is by the process of digestion that breaks down the complex substance of starch into simpler molecules of glucose, which are further metabolized into CO2 and water through the process of glycolysis. The human digestive tract starts at the mouth and ends at the anus.
In the Beginning
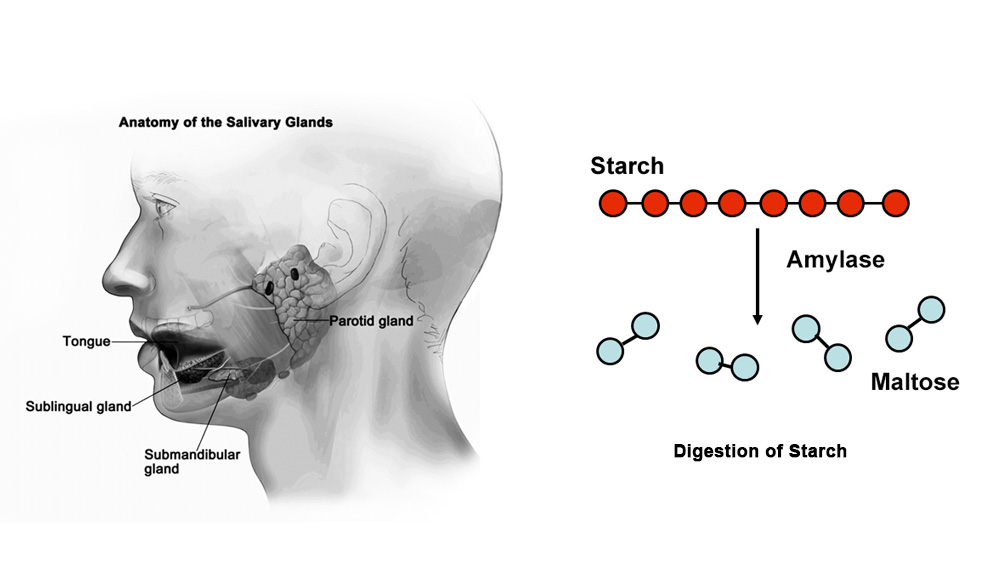
The digestion of the food starts as soon as we put food in our mouths. Our teeth cut the food into small pieces and the salivary glands secrete saliva that mixes with these food materials. The saliva contains an enzyme called salivary amylase which hydrolyses starch into maltose. The complete digestion of starch occurs only in the small intestine by the action of pancreatic amylase.
The activity of enzymes is strongly affected by several factors, such as temperature and pH.
The activity of enzymes is strongly affected by several factors, such as temperature and pH.
Effect of pH
The optimum pH for the enzymatic activity of salivary amylase ranges from 6 to 7. Above and below this range, the reaction rate reduces as enzymes get denatured. The enzyme salivary amylase is most active at pH 6.8. Our stomach has a high level of acidity which causes the salivary amylase to denature and change its shape. So the salivary amylase does not function once it enters the stomach.
How to test it?
The effect of pH on the activity of salivary amylase on starch can be studied by using the Iodine test. If we add saliva to starch, the salivary amylase present in saliva gradually acts on starch and converts it into maltose. Starch keeps on giving blue color with iodine till it is completely digested into maltose. At this point, no blue color is formed. This is the endpoint or achromic point.
Materials Required
Three series of test tubes having iodine solution in each, test tubes, pH tablets of 5, 6.8, and 8, the beaker containing water with the thermometer, 15 ml 1% starch solution + 3 ml 1% NaCl, saliva solution, droppers, Bunsen burner, and wire gauze.
Real Lab Procedure
- Take a beaker containing 15 ml of 1% starch solution + 3 ml of 1% NaCl solution.
- Divide and pour this solution into three test tubes and mark them as A, B, and C.
- Add pH tablet 5 into test tube A, pH tablet 6.8 into test tube B, and pH tablet 8 into test tube C.
- Now transfer experimental tubes A, B, and C into a beaker containing water and a thermometer for recording temperature. The temperature of this beaker is to be maintained at 37°C.
- Using a dropper, take 3 ml saliva solution and add 1 ml of the solution to each of the three test tubes.
- Immediately using a dropper, take few drops from experimental tube A and transfer this into the first series of test tubes having iodine solution.
- Similarly, do the same procedure for test tube B and test tube C and transfer the solution into the second and third series of test tubes having iodine solution.
- Note this time as zero minute reading.
- After an interval of 2 minutes, again take a drop from each tube and add to the iodine tubes, and note the change in color of iodine.
- Keep on repeating the experiment at an interval of every 2 minutes till the color of iodine does not change.
Results
pH 5 is acidic and pH 8 is alkaline, therefore salivary amylase did not act in these tubes. Whereas, the enzyme acted in the tube with pH 6.8 (i.e., slightly acidic) and digested the starch.
Note: If we add saliva on starch, the salivary amylase present in saliva gradually acts on starch and converts it into maltose. Starch keeps on giving blue color with iodine till it is completely digested into maltose. At this point, no blue color is formed. This is the endpoint or achromic point.
References
Books:
Laboratory Manual Biology for class XII – Published by NCERT.
Websites:
- http://www.britannica.com/science/amylase
- http://www.eng.umd.edu/~nsw/ench485/lab5.htm
- https://en.wikipedia.org/wiki/Amylase