Table of Contents
DimerCaprol
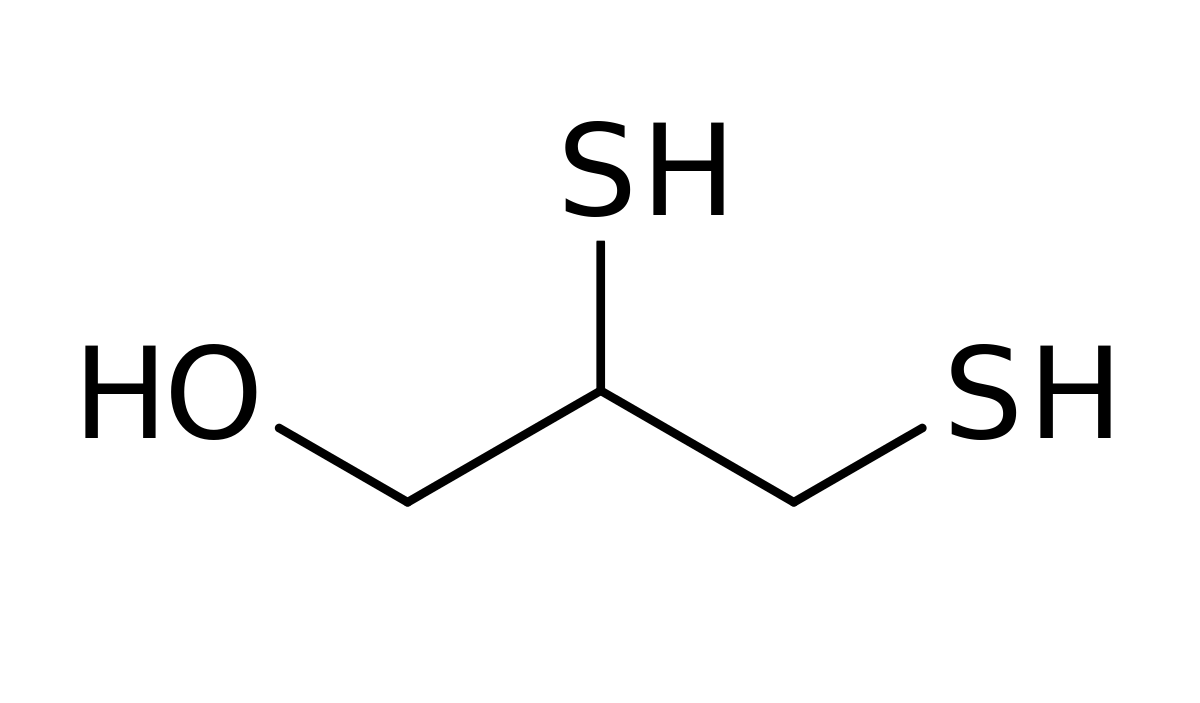
- Dimercaprol is (RS)-2,3-dimercaptopropanol.
- Dimercaprol contains not less than 98.5 percent w/w and not more than 101.5 percent w/w of C3H8OS2
Category.
Antidote in heavy metal poisoning; metal complexing agent.
Dose
By intramuscular injection, 2 to 3 mg per kg of body weight every 4 hours during the first day and subsequently, in accordance with the needs of the patient.
Description.
A clear, colorless, or slightly yellow liquid; odor, strong, characteristic, and alliaceous.
Identification
A. Dissolve 0.1 mI in 4 mI of water and to 2 mI of the solution add lead acetate solution; a yellow precipitate is obtained.
B. To 2 mI of the solution prepared for test Add 1 mI of 0.05M iodine; the color of iodine is immediately discharged.
C. In a ground-glass-stoppered tube suspend 0.6 g of sodium bismuthate, previously heated to 200° for 2 hours, in a mixture of 6 mI of water and 2.8 mI of a 10 percent w/w solution of phosphoric acid. Add 0.2 ml of the substance under examination, mix and allow to stand for 10 minutes shaking frequently. To 1 ml of the supernatant liquid add 5 mI of a 0.4 percent w/v solution of chronotropic acid sodium salt in sulphuric acid, mix, and heat for 15 minutes in a water-bath; a violet-red color is produced.
Tests
The appearance of the solution.
The substance under examination is clear (2.4.1), and not more intensely colored than reference solution BS6 orBYS6 (2.4.1).
pH
5.0 to 6.5, determined in a saturated solution.
Refractive index
1.568 to 1.574, determined at 20°.
Weight per ml
1.238 g to 1.240 g.
Iron
Ignite 2.0 g with 1 g of anhydrous sodium carbonate, cool, dissolve the residue in 15 ml of dilute hydrochloric acid and dilute to 45 mI with water; the resulting solution complies with the limit test for iron (20 ppm).
Halides.
To 2.0 g add 25 mI of 0.5 M ethanolic potassium hydroxide and heat under a reflux condenser for· 2 hours.
Remove the ethanol by evaporation in a current of warm air, add 20 mI of water, and cool. Add a mixture of 10 mI of strong hydrogen peroxide solution and 40 mI of water. Boil gently for 10 minutes; cool and filter rapidly. Add 10 mI of dilute nitric acid and 5 mI of 0.1 M silver nitrate and titrate with 0.1 M ammonium thiocyanate using ferric ammonium sulfate solution as an indicator. Repeat the operation without the substance under examination. The difference in the volumes of 0.1 M ammonium thiocyanate used in the two titrations is not more than 1.0 mI.
Assay
Weigh accurately about 0.1 g, dissolve in 40 mI of methanol and add 20 mI of 0.1 M hydrochloric acid and 50.0 mI of 0.05 M iodine. Allow to stand for 10 minutes and titrate with 0.1 M sodium thiosulphate. Repeat the operation
without the substance under examination. The difference between the titrations represents the amount of iodine required. 1mI of0.05M iodine is equivalent to 0.00621 g ofC3HsOSz.
Storage
Store protected from light in well-filled containers in a refrigerator (2° to 8°).






