Alkaline hydrolysis of primary alkyl halides is one of the best examples of SN2 reaction. In this ,nucleophile attacks from back side of the substrate , i.e. side opposite to the leaving group . Hence attachment of nucleophile and separation of leaving group takes place simultaneously to form transition state. further attack of nucleophile causes separation of leaving group to form product. In case of product ,configuration is found to get inverted as that of substrate. This inversion is known as Walden inversion and hundred percent product is formed by inversion.i.e.from dextrorotatory alkyl halide laevorotatory product is obtaine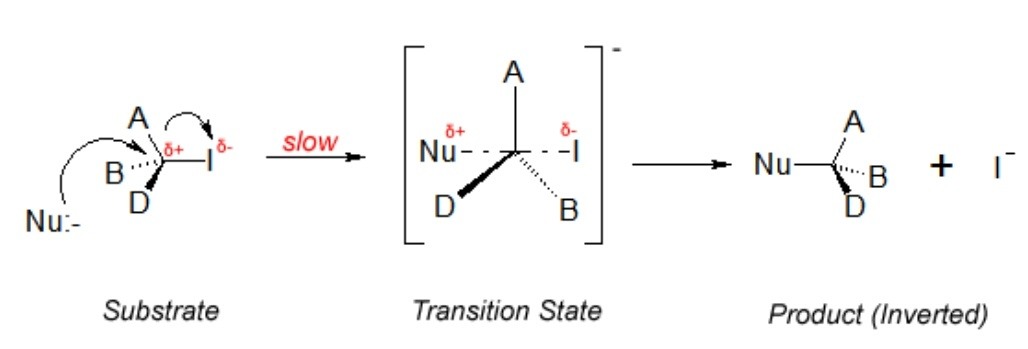
RecNotes Answered question March 16, 2021
